15 September 2023
CHO PHARMA INC. and T-E Med, Inc. Join Forces to Create a Powerful ADC Platform
Today, T-E Meds, Inc. and CHO PHARMA, INC., two leading biotechnology companies in the National Biotechnical Research Park, signed an MOU for co-developing advanced antibody-drug conjugates (ADCs) via complementary technologies.
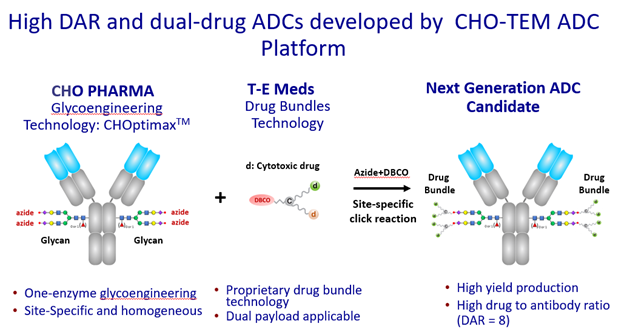
The innovative “drug bundle” technology of T-E Meds, Inc. employs the “multi-arm linker” platform to load drug molecules onto the “linking arms” and add functional groups, such as DBCO, for click chemistry reaction. The beauty of such drug bundles is when there are more than 2 linking arms, the modules can be designed to link the same or different drug molecules for various treatment options; meanwhile, the coupling arm still provides the functional group ready for click chemistry reaction. Moreover, the “drug bundles” are produced by solid-phase peptide synthesis with water-soluble amino acids and various PEGylated amino acids; therefore, they have built-in excellent solubility, which is difficult to achieve for ADCs in general.
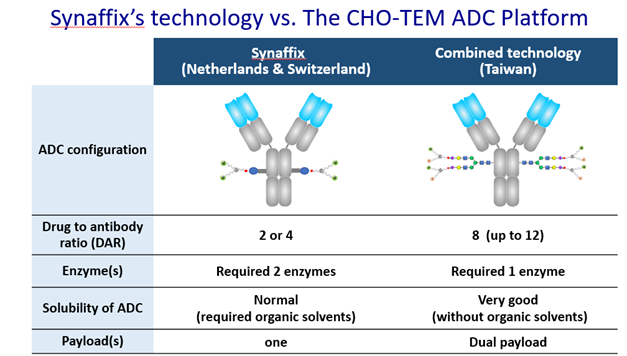
CHO PHARMA’s CHOptimax™ utilizes only 1 enzyme and takes only 1 step to create glycosite-specific ADCs with a drug-to-antibody ratio (DAR) of 4, significantly simplifying ADC production. Moreover, CHOptimax™ can specifically and rapidly glycoengineer Fc glycans on the antibodies to efficiently provide four sites to furnish functional groups for click chemistry reaction on any given antibody.
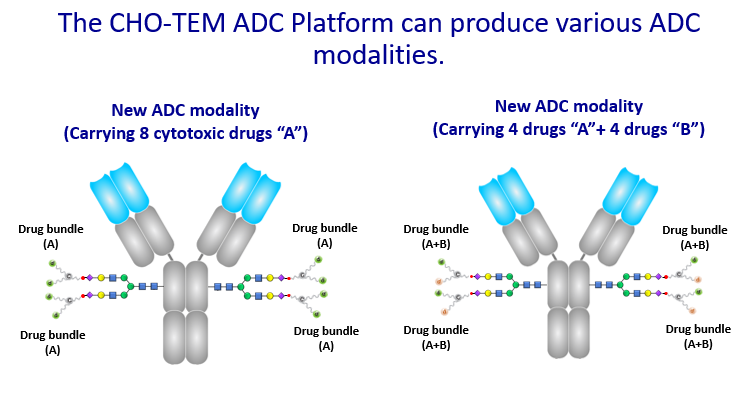
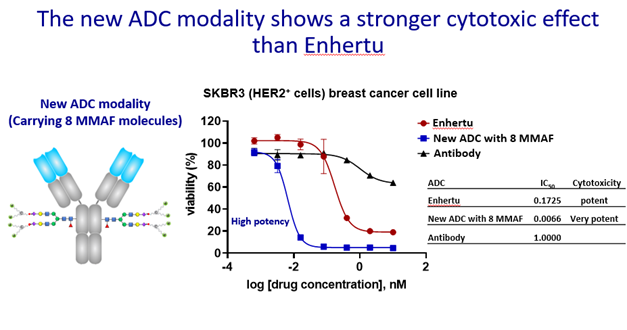
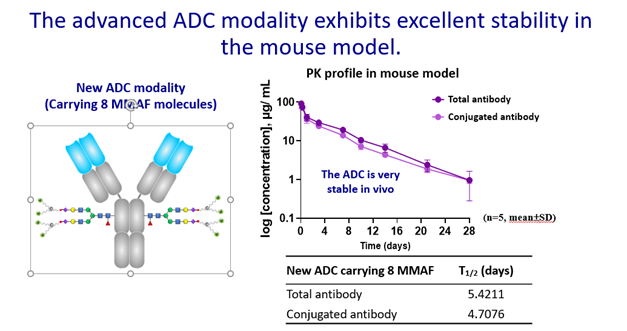
The advanced ADCs created by “drug bundle” technology and CHOptimax™ will be highly site-specific (yield > 90%) with a high drug-to-antibody ratio (DAR = 8). Remarkably, these 8 drugs can be all the same or 2 different kinds of 4 each (Please see illustration), making them perfect therapeutics for treating complex tumor types or dealing with cancer microenvironment. The in-vitro cell cytotoxicity study showed superior efficacy in killing cancer cells. The initial results also showed that these advanced ADCs are very stable in blood, unlike the conventional ADCs, which often exhibit problems such as precipitation or loss of payload when the DAR is high. In addition, the production of these advanced ADCs does not involve any organic solvent, which can alter antibodies, complicate the production process, and lower yields. In sum, the next-generation ADCs developed by CHO PHARMA, INC. and T-E Meds, Inc. would be more stable, more versatile, more effective, and made by a simpler production process.
The CHO PHARMA INC. and T-E Meds, Inc. collaboration has demonstrated POC (proof of concept); hence, both parties decided to enter into an MOU to solidify the partnership in optimizing the technology platform and creating more advanced, more complex ADC candidates. This strategic alliance involves cross-licensing complementary ADC technologies to co-develop next-generation, versatile ADCs that are site-specific and with high DAR. Both parties will also join business development endeavors in seeking international collaboration and licensing to the third party. The alliance will surely elevate both companies’ competitiveness and create shared profits.
Dr. Chao-Lung Chen, the Chairman of CHO PHARMA INC., stated, “In order to create more advanced ADCs, CHO PHARMA INC. has been actively searching for a technology to complement our proprietary CHOptimax™. This alliance with T-E Meds will help us achieve our goals and maintain our leadership in ADC development.”
Dr. Tse-Wen Chang, the Chairman of T-E Meds, Inc. said, “This collaboration between these two companies is more than 1 + 1 > 2. Our alliance fits so well. It’s like a completed jigsaw puzzle.”
About CHO PHARMA, INC.
CHO PHARMA, INC. was established in 2013 with headquarters located in the National Biotechnical Research Park, Nangang, Taiwan. CHO PHARMA has developed a glycoengineering platform, CHOptimax™, which utilizes 1 enzyme to modify specific IgG antibody Fc glycans in 1 simple step to increase antibody efficacy. Currently, CHO PHARMA focuses on utilizing CHOptimax™ to provide the best platform for ADC development and glycoengineer blockbuster antibody drugs into advanced, next-generation antibodies.
About T-E Meds, Inc.
T-E Meds, Inc. (established in 2022) is located in National Biotechnical Research Park, Nangang, Taiwan and a sister company of Immunework (established in 2014). Both companies are part of T-E Pharma Holding in the Cayman Islands. T-E Meds employs its proprietary “multi-arm linker” and “drug bundle” platforms in the development of ADCs and antibody radionuclide conjugates (ARCs) for improving drug safety and efficacy.
For more information, please contact:
CHO PHARMA INC., Dr. Chung-Yi Wu, President, Email: contact@chopharma.com.tw, 02-2655-8059
T-E Meds, Inc., Dr. Hsing-Mao Art Chu, CEO, Email: bd@temeds.com, 02-2651-2268

Illustrations: